Titanium(IV) Catecholate Family: Potential for Green Chemistry
Published:
Titanium(IV) Catecholate Complexes: Synthesis, Properties, and Prospects
Key Points
- Historical Context: Research suggests that Ti(IV) catecholate complexes, first well-characterized in the 1980s, are stable compounds with strong binding properties, making them promising for various applications.
- Synthesis and Structures: These complexes can likely be synthesized using titanium alkoxides or halides with catechol, forming both mononuclear and dinuclear structures, with recent studies exploring diverse architectures.
- Electronic and Optical Properties: Evidence leans toward these complexes exhibiting strong visible light absorption due to ligand-to-metal charge transfer (LMCT), with potential for tuning optical properties through ligand modifications.
- Applications: The complexes seem to hold potential in photocatalysis, photothermal conversion, and as alternatives to noble-metal catalysts, with patents indicating scalable synthesis methods.
- Uncertainty: While the field shows promise, the full scope of Ti(III) species stability and catalytic applications remains underexplored, suggesting a need for further research.
Overview
Titanium(IV) catecholate complexes are a fascinating class of compounds that combine the unique properties of titanium with the versatile chemistry of catecholate ligands. These complexes, derived from catechol (1,2-dihydroxybenzene), are known for their stability and ability to absorb visible light, making them candidates for applications in catalysis and materials science. Research from the 1980s onward has laid a foundation for understanding their synthesis and properties, with recent advancements highlighting their potential in modern technologies.
Synthesis and Structural Diversity
The synthesis of Ti(IV) catecholate complexes typically involves reacting titanium precursors, such as alkoxides or halides, with catechol. This process can produce both mononuclear and dinuclear complexes, with structures ranging from simple tris-catecholate monomers to complex oxo-bridged dimers. Recent studies have expanded the range of these complexes by varying ligands and solvents, suggesting a versatile platform for creating new materials.
Applications and Potential
The strong light absorption and redox properties of these complexes make them suitable for applications like photocatalysis and photothermal conversion. Their potential as cost-effective alternatives to noble-metal catalysts is particularly appealing due to titanium’s abundance and low toxicity. Additionally, patents indicate that these complexes can be synthesized on a large scale, which could facilitate their use in industrial applications like batteries and coatings.
Future Directions
While the field is promising, there are still gaps in understanding, particularly regarding the stability of Ti(III) species and the full extent of catalytic capabilities. Ongoing research into ligand modifications and higher-nuclearity clusters could unlock new possibilities, making these complexes a vibrant area for future exploration.
Comprehensive Report on Titanium(IV) Catecholate Complexes
Introduction to Catecholate Ligands
Catecholate ligands, derived from 1,2-dihydroxybenzene (catechol), are robust bidentate O,O′-donors that form highly stable chelates with various metal ions. These complexes are prevalent in bioinorganic chemistry, notably in iron(III)–catecholate siderophores, and are valued for their rich redox and photophysical properties [1]. Titanium(IV) binds catecholates with exceptional strength, often surpassing Fe(III) at physiological pH, due to the high Lewis acidity of Ti(IV) combined with the π-donor and redox-active nature of catecholates [1]. This interaction results in strong ligand-to-metal charge transfer (LMCT) absorptions, enabling Ti(IV) catecholate complexes to absorb visible light around 400 nm with intense LMCT bands (ε ~10^4 M⁻¹cm⁻¹). These characteristics have spurred interest in their use for photocatalysis, photothermal conversion, and as earth-abundant alternatives to noble-metal catalysts [1].
Early Syntheses and Known Structures
The first well-characterized Ti(IV) tris-catecholate complexes emerged in the 1980s [1]. A straightforward synthesis involves reacting titanium alkoxides or halides with excess catechol. For instance, Ti(O^iPr)_4 combines with three equivalents of catechol (H₂Cat) in an inert solvent to yield the diprotonated tris(catecholate) [H₂Ti(Cat)_3] (1H₂), which is isolated as a solid containing two acidic protons on two catecholate ligands. Treating 1H₂ with a base like triethylamine (NEt₃) produces the di-ammonium salt [(HNEt₃)_2][Ti(Cat)_3], featuring the anionic [Ti(Cat)_3]^2– unit [1]. These synthetic routes are illustrated in Figure 1.
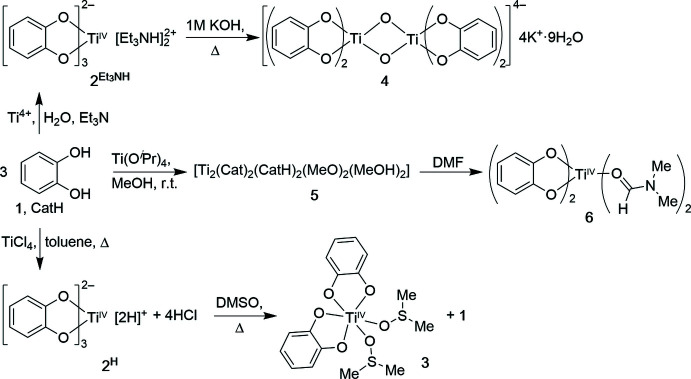
Figure 1. Synthetic routes to Ti(IV)–catecholate complexes reported in the literature [1]. The diprotonated tris(catecholate) [H₂Ti(Cat)_3] (2H) is formed by mixing Ti precursors with catechol; deprotonation (with base) yields the salt (HNEt₃)_2[Ti(Cat)_3] (3). Heating [H₂Ti(Cat)_3] in DMSO displaces one catechol to give the neutral bis(catecholate) species 3 (complex described below) [6]. Anionic dinuclear species like 4 with [Ti₂O₂(Cat)_4]^4– cores arise via condensation (base and heat) [2]. (CatH = catechol; Cat = catecholate anion.)
X-ray crystallography reveals that neutral [H₂Ti(Cat)_3] (1H₂) can exist as a discrete monomer with a Ti^IVO₆ core or condense into dinuclear oxo-bridged complexes [1]. One study demonstrated that grinding [H₂Ti(Cat)_3] with SrCO₃ or BaCO₃ produces solids Sr[Ti(Cat)_3]_3·H₂O and Ba[Ti(Cat)_3]_3·H₂O, which, upon recrystallization, yield water-soluble salts [Sr(H₂O)_5]_2[Ti₂O₂(Cat)_4]·6H₂O and [Ba(H₂O)_4(MeOH)]_2[Ti₂O₂(Cat)_4]·solvent [1]. Single-crystal X-ray data indicate that these crystals contain dinuclear [Ti₂O₂(Cat)_4]^4– cores, where two [TiCat₂] units are linked by two μ₂-O^2– bridges, rather than isolated [Ti(Cat)_3]^2– units [1].
Recent advancements have introduced new architectures by varying metal precursors, solvents, and ligand substituents. Bazhenova et al. [2] reported four complexes (1–4) synthesized by treating Ti(OCH₃)_4 with catechol or 4-tert-butylcatechol in methanol. Complexes 1 and 2 are neutral dimers (Ti₂) with one bridging and one terminal catecholate per dimer. Complex 3 is a rare neutral mononuclear Ti complex with two catecholate ligands and two DMF ligands. Complex 4 is an anionic heterometallic assembly [{Na(dme)Ti(Cat)_3}_2]^2– with Na^+ countercations, where each Ti is octahedral with three catecholates exhibiting varied binding modes [2]. These findings highlight the structural versatility of Ti–catecholate chemistry.
Electronic Structure and Spectroscopy
As a d^0 metal, Titanium(IV) relies on LMCT for its visible/UV absorption, where catecholate π orbitals donate to empty Ti 3d orbitals, producing intense bands in the near-UV/blue region (350–450 nm) [3]. UV–vis spectra of Ti(Cat)_3^2– show strong absorptions, and TiO₂ nanoparticles functionalized with catechols display LMCT tails in the visible region [3]. These transitions are responsible for the complexes’ visible color.
Recent research has quantified how catecholate ligation reduces the band gap of Ti-oxo materials. Lv et al. [4] synthesized Ti₆ and Ti₈ oxo-clusters with catecholate ligands, achieving a band gap reduction to ~2.1 eV (absorption edge ~590–670 nm) [4]. Density-functional theory (DFT) calculations confirmed that catecholate π orbitals elevate the HOMO, facilitating low-energy LMCT [4]. Hou et al. [5] reported a groundbreaking “all-catecholate” Ti₁₆O₈(OH)_8(Cat)_20 cluster (Ti16), which is black, absorbing across the entire visible spectrum due to its ultralow band gap and exceptional stability [5]. This cluster, with 20 catecholate ligands enveloping a Ti₁₆O₈(OH)_8 core, marks the first high-nuclearity Ti-oxo cluster fully protected by catecholates [5].
The redox chemistry of Ti(IV)/Ti(III) is pivotal, with catecholates acting as non-innocent ligands where reduction may occur at the metal or ligand [3]. Creutz et al. [3] found that Ti(IV)/Ti(III) potentials in Ti(cat)_3 complexes are relatively insensitive to catechol substitution, with potentials ranging from –0.5 to –1.0 V vs. NHE, enabling Ti(III) generation under strong reducing conditions [3].
Mononuclear Bis- and Tris-Catecholate Complexes
Most Ti(IV) catecholate complexes feature tris-catecholate coordination (TiO₆ with three O₂C₆H₄ ligands), but stable bis-catecholate species are challenging to isolate due to the ligand’s charge [6]. Hewage et al. [6] reported a rare neutral mononuclear Ti(IV) bis(catecholate) complex, [Ti(Cat)_2(DMSO)_2], formed by refluxing [H₂Ti(Cat)_3] in DMSO, displacing one catechol with two DMSO molecules [6]. This complex, only the second of its kind after [Ti(Cat)_2(DMF)_2], exhibits distorted octahedral geometry [6]. Mixed-ligand bis-catecholate complexes can incorporate donor solvents or other ligands, enhancing stability [1]. The exceptional stability of Ti(IV) catecholates, with formation constants ~10^20 or higher, renders Ti(cat)_3 complexes inert to ligand displacement in water [1].
Dinuclear (Bi-Metallic) Bridged Complexes
Ti(IV) catecholates readily form dinuclear structures, often with oxo or catecholate bridges [1]. The [Ti₂O₂(Cat)_4]^4– core, a bis(μ-oxo) bridged dimer, forms spontaneously under mild base or heat, with each Ti chelated by two catecholates and linked by two O^2– bridges [1]. Bazhenova et al. [2] described dimers where one catecholate bridges two Ti atoms in a μ₂-(O,O′,O′) fashion [2]. Mixed-metal complexes, such as [Na(dme)Ti(Cat)_3]_2[Na(dme)(MeOH)]_2, feature alkali or alkaline-earth cations coordinating catecholate oxygens, forming extended networks [2]. These heterometallic assemblies underscore the bridging potential of Ti–catecholate systems.
Photochemical and Catalytic Applications
The low-energy LMCT states of Ti(IV) catecholate complexes enable rich photochemistry, potentially generating catecholato radicals or Ti(III) species [3]. Catecholate–TiO₂ surface complexes sensitize TiO₂ in the visible, supporting photocurrent generation up to ~550 nm [3]. Soluble Ti(cat)_3 complexes are also explored as visible-light absorbers [3]. In catalysis, Bazhenova et al. [2] demonstrated that complexes 1–4 catalyze acetylene hydrogenation to ethylene using Na/Hg, suggesting multi-electron catalytic cycles involving Ti(III)/Ti(IV) transitions [2]. While not yet widely applied, their light absorption and redox properties indicate potential in photoredox or electrocatalytic systems [2, 3]. Catechol–TiO₂ composites in dye-sensitized solar cells and H₂ generation suggest homogeneous Ti(catecholate)₃ analogues could be viable [3].
Prospects: New Complexes and Ligand Variations
Ligand Modification
Substituted catechols (e.g., 3,5-di-tert-butylcatechol, 4-t-butylcatechol) can tune sterics, electronics, and solubility [1]. Electron-donating groups may redshift LMCT bands, while sulfonated or phosphonated catecholates could enhance water solubility or support binding [4]. Mixed-ligand systems with O-donors like acetylacetonate may yield stable heteroleptic complexes [1].
Higher-Nuclearity and Cluster Assembly
The Ti₁₆ cluster demonstrates the feasibility of templating large Ti–O clusters with catechols [5]. Bifunctional catechols or polyols like gallic acid could create 1D or 2D networks [5]. Mixed-metal clusters with Fe or Mn could introduce magnetic or multielectron redox properties [2].
Ti(III) Catecholate Species
Reducing Ti(IV)–catecholates to Ti(III) is underexplored but promising [3]. Stable Ti(III) complexes could serve as single-electron catalysts, while transient Ti(III)(cat) intermediates may contribute to photocatalysis [3].
Semi-Synthetic Analogues
Inspired by siderophores, grafting catecholate–Ti onto polymers or nanoparticles could yield materials for dye removal or catalysis [1]. Embedding Ti(IV)–catecholate units in MOFs could produce light-harvesting porous materials [1].
Potential for Scale-Up and Applications
Ti(IV) catecholates are attractive due to titanium’s abundance and low toxicity [1]. Their strong visible absorption supports solar-driven applications, with the Ti₁₆ cluster showing exceptional photothermal conversion [5]. They are proposed for organic flow batteries due to high solubility and reversible redox [7]. In pigment or coating industries, they could serve as UV-blockers or colorants [1]. Patents like Lockheed-Martin US10,377,687 outline scalable synthesis routes using titanium tetrachloride or alkoxides, producing water-soluble salts [7]. Hydrolysis control remains a challenge, but kilogram-scale production is feasible with inert handling [7].
Conclusion
Ti(IV) tris(catecholate) complexes bridge inorganic and organic chemistry, with a history rooted in the 1980s and expanding into advanced materials [1]. Their strong visible-light absorption, tunable redox, and structural versatility (monomers, dimers, polymers) highlight their potential [1]. Recent developments, like the Ti₁₆ cluster, and catalytic demonstrations underscore untapped opportunities [5]. Novel ligand designs and heterometallic assemblies could rival noble-metal systems, leveraging titanium’s cost-effectiveness for applications in solar fuels, sensors, and electronics [1].
References
[1] B. A. Borgias, S. R. Cooper, Y. B. Koh, K. N. Raymond, Inorg. Chem. 1984, 23, 1009-1016. https://doi.org/10.1021/ic00176a005
[2] T. A. Bazhenova, N. V. Kovaleva, G. K. Fukin, G. N. Petrova, D. A. Kuznetsov, Eur. J. Inorg. Chem. 2016, 5215-5221. https://doi.org/10.1002/ejic.201600804
[3] C. Creutz, M. Chou, Inorg. Chem. 2008, 47, 3509-3514. https://doi.org/10.1021/ic701687k
[4] H. Lv, H. Li, G. Zou, Y. Cui, Y. Huang, Y. Fan, Dalton Trans. 2018, 47, 8158-8163. https://doi.org/10.1039/c8dt01844h
[5] J. Hou, N. Huang, D. Acharya, Y. Liu, J. Zhu, J. Teng, Z. Wang, K. Qu, X. Zhang, D. Sun, Chem. Sci. 2024, 15, 2655-2664. https://doi.org/10.1039/d3sc05617a
[6] D. Hewage, K. R. Leopold, A. J. Lees, Acta Cryst. 2022, E78, 347-351. https://doi.org/10.1107/S2056989022002088
[7] Lockheed-Martin US10,377,687. https://patents.google.com/patent/US10377687B2/en
Table: Summary of Key Ti(IV) Catecholate Complexes
| Complex Type | Description | Key Features | Reference |
|---|---|---|---|
| [H₂Ti(Cat)_3] | Diprotonated tris(catecholate) | Monomeric, Ti^IVO₆ core, stable in solid form | [1] |
| [(HNEt₃)_2][Ti(Cat)_3] | Di-ammonium salt | Anionic [Ti(Cat)_3]^2– unit, formed by deprotonation | [1] |
| [Ti₂O₂(Cat)_4]^4– | Dinuclear bis(μ-oxo) | Two Ti centers bridged by two O^2–, each with two catecholates | [1] |
| Ti₂ Dimers (Complexes 1, 2) | Neutral dimers | One bridging and one terminal catecholate per Ti | [2] |
| [Ti(Cat)_2(DMSO)_2] | Neutral mononuclear bis(catecholate) | Two catecholates, two DMSO ligands, octahedral geometry | [6] |
| [{Na(dme)Ti(Cat)_3}_2]^2– | Heterometallic assembly | Octahedral Ti with three catecholates, Na^+ bridging | [2] |
| Ti₁₆O₈(OH)_8(Cat)_20 | All-catecholate cluster | Black, ultralow band gap, high stability | [5] |

